Biomass, which is useful as a supplement or replacement for petroleum, is processed in biorefineries and can be used as fuels or starting materials for the production of chemicals. However, the high oxygen content of many biomass components poses a problem. In the journal Angewandte Chemie, German researchers have now introduced a process based on a tandem reaction that could reduce the oxygen content of both lignin and bio-oils under unusually mild conditions.
In principle, a biorefinery works like a petroleum refinery: a complex mixture of substances is separated into individual components and partially refined - chemically converted into other, more useful compounds. Lignin is one of the major components of biomass and is a byproduct produced in large amounts by the paper and pulp industry. Even in the biorefineries, no better application than its use as solid fuel has been found so far.
Lignin is a heterogeneous group of phenolic macromolecules. Phenols are aromatic six-membered rings of carbon atoms with some number of alcohol (OH) functional groups. The depolymerization of lignin to form low-boiling arenes - oxygen-free aromatics - instead of high-boiling phenols would represent a great simplification of conventional refining processes.
Unfortunately, phenolic OH groups are not so easy to cleave because the bond between the phenolic oxygen atom and the aromatic ring is very strong. Previous methods have been forced to use a detour involving derivatization, in which an electron-withdrawing group is attached to the oxygen atom to weaken its bond to the carbon atom. This allows the bond to be broken catalytically in the presence of hydrogen. This process is not practical on a large scale because it also results in large quantities of nonrecyclable byproducts.
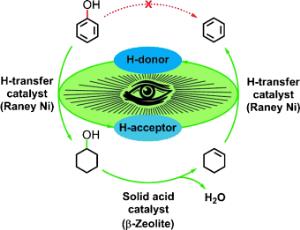
Xingyu Wang and Roberto Rinaldi at the Max Planck Institute for Coal Research in Muelheim (Ruhr, Germany) have now introduced a new, one-step process for the depolymerization of lignin with simultaneous, highly selective conversion of phenols to arenes. In contrast to other processes, this one works under mild conditions and with no formation of derivatives.
Their trick is the clever linking of three reactions into a reaction cascade that consists of an initiation reaction and a tandem reaction. The catalysts are Raney nickel and a zeolite. Because hydrogen gas would disrupt this reaction system, hydrogen must be introduced to the system in another way: 2-propanol is first used to provide hydrogen for the initiation reaction. The cyclohexene formed in phase 1 of the tandem reaction then provides the hydrogen for phase 2 of the tandem reaction.
This new method is a highly promising starting point for the development of innovative industrial processes for refining lignin and the phenolic fraction of bio-oils under mild conditions. The conversion of lignin into simple arenes offers a novel avenue for boosting lignocellulosic biorefinery.
About the Author
Dr. Roberto Rinaldi is an independent group leader at the Max Planck Institute for Coal Research, and has been working in the area of bioenergy for over 6 years. In 2010, he received one of the most prestigious awards in Europe: the Sofja Kovalevskaja Award, given by the Alexander von Humboldt Foundation, to start his independent research group in Muelheim in the field of catalytic valorization of lignin.
Further Information:
Xingyu Wang, Dr. Roberto Rinaldi:
A Route for Lignin and Bio-Oil Conversion: Dehydroxylation of Phenols into Arenes by Catalytic Tandem Reactions.
In: Angewandte Chemie International Edition; published online 12 September 2013, DOI 10.1002/anie.201304776
Source: Angewandte Chemie International Edition, press release 37/2013
Last update: 29.09.2013
Perma link: https://www.internetchemistry.com/news/2013/sep13/biomass-tandem.php
More chemistry: index | chemicals | lab equipment | job vacancies | sitemap
Internetchemistry: home | about | contact | imprint | privacy
© 1996 - 2023 Internetchemistry
