Carbon monoxide is highly toxic since it blocks the binding site for oxygen in hemoglobin. This very principle - a porphyrin ring with a central iron or cobalt atom that the poisonous gas attaches to - can be used to implement sensors to warn against carbon monoxide.
Physicists headed by Professor Johannes Barth from the Technische Universitaet Muenchen (TUM) have, in collaboration with theorists in Lyon and Barcelona, deciphered the mechanism for binding of gas molecules to iron and cobalt porphyrins. They present the unexpected phenomena they discovered in the current issue of Nature Chemistry, including the first images.
The mechanism for binding oxygen to metalloporphyrins is a vital process for oxygen-breathing organisms. Understanding how small gas molecules are chemically bound to the complexed metal centers is also important in catalysis or the implementation of chemical sensors. When investigating these binding mechanisms, scientists use porphyrin rings with a central cobalt or iron atom. They coat a copper or silver support surface with these substances.
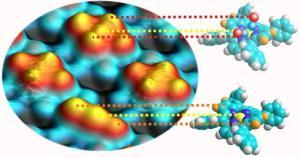
An important characteristic of porphyrins is their conformational flexibility. Recent research has shown that each specific geometric configuration of the metalloporphyrins has a distinct influence on their functionality. In line with the current state of research, the scientists expected only a single CO molecule to bind axial to the central metallic atom. However, detailed scanning tunnel microscopy experiments by Knud Seifert revealed that, in fact, two gas molecules dock between the central metallic atom and the two opposite nitrogen atoms. Decisive is the saddle shape of the porphyrin molecules in which the gas molecules assume the position of the rider.
The significance of the saddle geometry became apparent in model calculations done by Marie-Laure Bocquet from the University of Lyon. Her analysis helped the researchers understand the novel binding mode in detail. She also showed that the shape of the molecular saddle remains practically unchanged, even after the two gas molecules bind to the porphyrin.
The porphyrins reacted very differently when the researchers replaced the carbon monoxide with stronger-binding nitrogen monoxide. As expected, this binds directly to the central atom, albeit only a single molecule fits in each porphyrin ring. This has a significant effect on the electronic structure of the carrier molecule and the characteristic saddle becomes flattened. Thus, the porphyrin reacts very differently to different kinds of gas - a result that is relevant for potential applications, like sensors.
Dr. Willi Auwaerter, one of the authors, is thrilled: "New is that we actually saw, for the first time, the mechanism on a molecular level. We even can selectively move individual gas molecules from one porphyrin to another." The team aims to explain the physical and chemical processes on surfaces and in nanostructures. Once these fundamental questions are answered they will take on new challenges: How big is the influence of the central atom? How does the binding change in planar conformations? How can such systems be utilized to implement catalyzers and sensors through controlled charge transfers?
The research was funded by the Deutsche Forschungsgemeinschaft (Excellence Cluster "Munich-Centre for Advanced Photonics" (MAP)), the TUM Institute for Advanced Study, the European Research Council (ERC Advanced Grant MolArt), as well as the Spanish Ministerio de Ciencia E Innovacion. The Leibniz Rechenzentrum of the Bayerische Akademie der Wissenschaften provided computing time. The research group of Professor Barth is member of the Catalysis Research Center (CRC) of the TUM.
Further Information:
Knud Seufert, Marie-Laure Bocquet, Willi Auwärter, Alexander Weber-Bargioni, Joachim Reichert, Nicolas Lorente und Johannes V. Barth:
Cis-dicarbonyl binding at cobalt and iron porphyrins with saddle-shape conformation.
In: Nature Chemistry; published online: 09 January 2011, DOI 10.1038/nchem.956
Knud Seufert, Willi Auwrter, and Johannes V. Barth:
Discriminative Response of Surface-Confined Metalloporphyrin Molecules to Carbon and Nitrogen Monoxide.
In: Journal of the American Chemical Society; 2010, 132 (51), pp 18141 - 18146, DOI 10.1021/ja1054884
Source: Munich-Centre for Advanced Photonics, MAP, Germany
Last update: 19.01.2011.
Perma link: https://www.internetchemistry.com/news/2011/jan11/carbon-monoxide-binding-observation.php
© 1996 - 2023 Internetchemistry