|
Chemistry News Archive March 2011
|
Chemistry News March 2011
News of the year 2011 in the fields of chemistry and chemistry-related topics like biochemistry, nantechnology, medicinal chemistry etc.
Main focus: press releases, scientific research results and summaries of chemistry articles, that are published in chemistry journals.
Please send us a eMail to publish your press release here!
|
|
| | | Chemistry: |  |
 |
Nanometer-scale gold particles are currently intensively investigated for possible applications in catalysis, sensing, photonics, biolabeling, drug carriers and molecular electronics. Image: This is an atomistic model of the Au102(p-MBA)44 particle. Gold: yellow, sulfur: orange, carbon: green, oxygen:r ed, hydrogen: white [Credit: Academy of Finland].
|
|
New method could improve economics of sweetening natural gas. ASSR could reduce amount of heat needed in purification process.
|
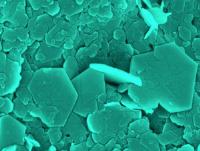 |
Green sludge can protect groundwater from radioactive contamination. Image: University of Copenhagen chemists have shown that green rust is capable of capturing and containing almost any kind of pollution in soil due to its extreme chemical reactivity [Credit: Bo C. Christiansen/University of Copenhagen].
|
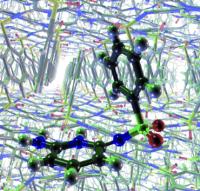 |
Tough crystal nut cracked: Correct prediction of all three known crystal structures of a sulfonimide. Image: Past failures to predict the polymorphs of a sulfonimide using molecular mechanics have led to speculation that crystal-structure prediction may be of limited use owing to the kinetic nature of crystallization. An approach based on quantum mechanics now successfully predicts the three known polymorphs of this compound [Credit: Angewandte Chemie International Edition].
|
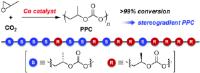 |
Sorted building blocks: synthesis of stereogradient poly(propylene carbonate) [Image credit: Angewandte Chemie]
|
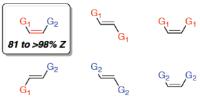 |
New method for preparation of high-energy carbon-carbon double bonds. BC and MIT researchers reveal power of new catalyst class and olefin metathesis process. The described catalyst controlled Z-selective cross-metathesis (CM) yields up to 98% Z-isomers [Credit: Nature].
|
| | | Physics: | 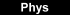 |
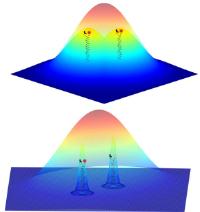 |
Novel high-resolution methods in fluorescence microscopy. Heidelberg scientists employ chemical reaction for light-independent switching of fluorescent probes. Image: The fluorescent signals from two nearby objects are superimposed by diffraction and imaged as a single feature. The ability to image individual probes separately means that their positions can be determined much more accurately to reconstruct the whole structure [Credit: University of Heidelberg].
|
|
Accurate measurement of radioactive thoron possible at last.
|
 |
Glowing spirals: chemical scaffolds guide living cells into precisely defined three-dimensional patterns. Image: Falling into line - A method for the precise generation of durable 3D chemical patterns within stationary media was used to direct the chemotactic self-organization of living cells [Credit: Angewandte Chemie].
|
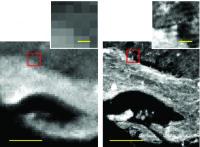 |
New imaging technique provides rapid, high-definition chemistry. Chemical images now much more detailed. Image: IRENI-generated images (right) are 100 times less pixelated than in those from conventional infrared imaging (left). Using multiple beams from a synchrotron provided made the difference, providing enough light to obtain a detailed image of the sample [Credit: Carol Hirshmugl/Michael Naase].
|
| | | Biochemistry: |  |
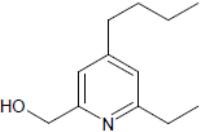 |
Compound useful for studying birth defects may also have anti-tumor properties. Image: Structure of Heterotaxin, a novel pyridine analoga that inhibits TGF-ß-dependent left-right asymmetric gene expression.
|
|
Researchers develop curious snapshot of powerful retinal pigment and its partners. Three's not a crowd when it comes to triggering the senses and other physiological functions.
|
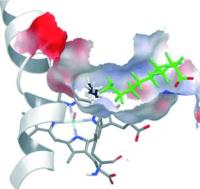 |
Extra guest molecule in an enzyme’s binding pocket enables methane oxidation. Image: A new spin - The addition of chemically inert perfluoro carboxylic acids (green; see picture) to P450 enzymes results in dramatic activation of their catalytic activity as a result of the conversion of the Fe/heme from a low-spin to a high-spin state, and the reduction of the binding-pocket size. Together these effects allow otherwise inert substrates such as propane and even methane to be oxidized [Credit: Angewandte Chemie International Edition].
|
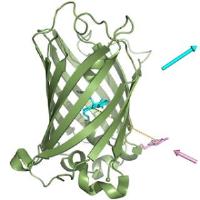 |
Synthetic biology: TUM researchers develop novel kind of fluorescent protein. Image: JACS - Biosynthesis of a fluorescent protein with extreme pseudo-Stokes shift by introducing a genetically encoded non-natural amino acid outside the fluorophore. A novel kind of fluorescent protein relying on the intramolecular interplay between two different fluorophores, one of chemical origin and one of biological origin, was developed [Credit: A. Skerra / TUM].
|
 |
Production of mustard oils: During the evolution of plants of the mustard family a leucine producing enzyme mutated into an enzyme that protects plants against herbivores. Image: Plants of the mustard family, such as cabbage, produce glucosinolates that help to fend off herbivorous insects by reacting as part of “mustard oil bombs” [Credit: MPI for Chemical Ecology/A. Schneider].
|
 |
'Lost' samples from famous origin of life researcher could send the search for Earth's first life in a new direction. Image: Preserved samples from a 1958 experiment done by "primordial soup" pioneer Stanley Miller contain amino acids created by the experiment [Credit: Scripps Institution of Oceanography, UC San Diego].
|
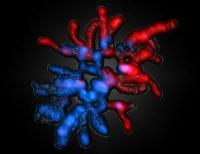 |
Saarbrücker researchers show how parental genomes are reprogrammed at the start of life in mammals. Image: Female and male-derived chromosomes of a fertilized egg of a mouse [Credit: Saarland University, Prof. Walter].
|
| | | Chemistry and Medicine: |  |
|
Researcher lists more than 4,000 components of blood chemistry.
|
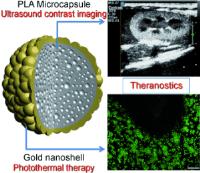 |
Double strike to fight cancer - combined diagnosis and treatment of tumors: photothermically activated ultrasound contrast agent. Image: The combination of electrostatic deposition of gold nanoparticles onto microcapsules and a surface seeding method results in the formation of gold nanoshells (see picture). This nano/micro composite is able to operate as a theranostic agent for both contrast-enhanced ultrasonic imaging (diagnostic) and photohyperthermia (therapeutic), and thus holds a great potential for photothermal therapy in cancer treatment [Credit: Angewandte Chemie International Edition].
|
| | | Chemistry and Nanotechnology: | 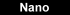 |
 |
Next-generation chemical mapping on the nanoscale ... [Image Credit: Nano Letters]
|
 |
Twinkle, twinkle, quantum dot - new particles can change colors and tag molecules. Image: Researchers have invented fluorescent nano-particles that change color to tag molecules under the microscope [Credit: Gang Ruan, courtesy of Ohio State University].
|
| | | Chemistry and Materials: |  |
 |
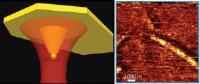
Berkeley Lab scientists achieve breakthrough in nanocomposite for high-capacity hydrogen storage. Image: This schematic shows high-capacity magnesium nanocrystals encapsulated in a gas-barrier polymer matrix to create a new and revolutionary hydrogen storage composite material [Credit: Jeff Urban].
|
 |
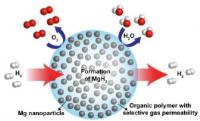
Upgrading the vanadium redox battery: New electrolyte mix increases energy storage by 70 percent. Image: This artist's rendering of an upgraded vanadium redox battery shows how using both hydrochloric and sulfuric acids in the electrolyte significantly improves the battery's performance and could also improve the electric grid’s reliability and help connect more wind turbines and solar panels to the grid [Credit: Pacific Northwest National Laboratory].
|
| | | More News (open access): |
Greener process for key ingredient for everything from
paint to diapers
Scientists are reporting discovery of an
environmentally friendly way to make a key industrial
material - used in products ranging from paints to diapers -
from a renewable raw material without touching the
traditional pricey and increasingly scarce petroleum-based
starting material. Their report on a new catalyst for making
acrylic acid appears in ACS Catalysis, the newest in the
American Chemical Society’s suite of 39 peer-reviewed
scientific journals. Weijie Ji, Chak-Tong
Au, and colleagues note that acrylic acid is essential for
making paints, adhesives, textiles, leather treatments, and
hundreds of other products. Global demand for the colorless
liquid totals about 4 million tons annually. Acrylic acid is
typically made from propylene obtained from petroleum. With
prices rising, manufacturers have been seeking alternative
ways of making acrylic acid without buying propylene. One
possibility involves making it from lactic acid. But current
processes for using lactic acid are inefficient, less
selective, and require higher temperatures and the
accompanying high inputs of energy. The
scientists’ potential solution is a new catalyst that can
convert lactic acid into acrylic acid more efficiently.
Lactic acid is a classic renewable starting material,
produced by bacteria growing in vats of biomass such as
glucose and starch from plants. In laboratory studies, the
scientists showed that the new catalyst can convert lactic
acid to acrylic acid more selectively at lower temperatures.
This could mean better use of lactic acid, lower fuel
consumption, and less impact on the environment, the
scientists suggest. ACS Catalysis:
"Efficient Acrylic Acid Production through Bio Lactic Acid
Dehydration over NaY Zeolite Modified by Alkali Phosphates"
[ACS Catal., 2011, 1 (1), pp 32–41; DOI: 10.1021/cs100047p]. |
Does selenium prevent cancer? It may
depend on which form people take
Scientists are reporting that the
controversy surrounding whether selenium can fight cancer in
humans might come down to which form of the essential
micronutrient people take. It turns out that not all
"seleniums" are the same - the
researchers found that one type of selenium supplement may
produce a possible cancer-preventing substance more
efficiently than another form of selenium in human cancer
cells. Their study appears in the ACS' journal Biochemistry.
Hugh Harris and colleagues note that
although the Nutritional Prevention of Cancer clinical trial
showed that selenium reduced the risk of cancer, a later
study called the Selenium and Vitamin E Cancer Prevention
Trial did not show a benefit. A major difference between the
trials was the form of selenium that was used. To find out
whether different types of selenium have different
chemopreventive properties, the researchers studied how two
forms - SeMet and MeSeCys - are processed in human lung cancer cells.
The researchers found that MeSeCys killed
more lung cancer cells than SeMet did. Also, lung cancer
cells treated with MeSeCys processed the selenium
differently than than cells treated with SeMet. They say
that these findings could explain why studies on the health
benefits of selenium sometimes have conflicting results.
The authors acknowledge funding from the
Australian Research Council.
ACS Biochemistry: "Uptake,
Distribution, and Speciation of Selenoamino Acids by Human
Cancer Cells: X-ray Absorption and Fluorescence Methods" [January 2011, 50 (10), pp 1641–1650]. |
New 'dissolvable tobacco' products may increase risk of mouth disease
The first study to analyze the complex
ingredients in the new genre of dissolvable tobacco products
has concluded that these pop-into-the-mouth replacements for
cigarettes in places where smoking is banned have the
potential to cause mouth diseases and other problems. The
report appears in ACS's Journal of Agricultural and Food
Chemistry. John V. Goodpaster and
colleagues point out that the first dissolvable tobacco
products went on sale in 2009 in test markets in
Indianapolis, Ind., Columbus, Ohio, and Portland, Ore. The
products contain finely-ground tobacco and other ingredients
processed into pellet, stick, and strip forms that are
advertised as smoke and spit-free. Health officials are
concerned about whether the products, which dissolve inside
the mouth near the lips and gums, are in fact a safer
alternative to cigarette smoking. Goodpaster and colleagues
note the possibility that children may be accidentally
poisoned by the nicotine in these products. "The packaging
and design of the dissolvables may also appeal to children,
and some dissolvables, such as Orbs, may be mistaken for
candy," the report states. The
researchers' analysis found that the products contain mainly
nicotine and a variety of flavoring ingredients, sweeteners,
and binders. They note abundant scientific evidence about
the potential adverse health effects of nicotine, including
those involving the teeth and gums. Other ingredients in
dissolvables have the potential to increase the risk of
tooth decay and one, coumarin, has been banned as a
flavoring agent in food because of its link to a risk of
liver damage. "The results presented here
are the first to reveal the complexity of dissolvable
tobacco products and may be used to assess potential health
effects," said Goodpaster, noting that it is "therefore
important to understand some of the potential toxicological
effects of some of the ingredients of these products."
Nicotine in particular, he noted, is a toxic substance
linked to the development of oral cancers and gum disease.
Journal of Agricultural and Food Chemistry: "Chemical
Characterization of Dissolvable Tobacco Products Promoted To
Reduce Harm" [J. Agric. Food Chem., 2011, 59 (6),
pp 2745–2751]. |
Laser beam makes cells 'breathe in' water and potentially anti-cancer drugs
Shining a laser light on cells and then
clicking off the light makes the cells "breathe in"
surrounding water, providing a potentially powerful delivery
system for chemotherapy drugs, as well as a non-invasive way
to target anti-Alzheimer's medicines to the brain. That's
the conclusion of a report in ACS's The Journal of Physical
Chemistry Letters. Andrei Sommer's group,
with Emad Aziz and colleagues note using this technique
before to force cancer cells to sip up anti-cancer drugs and
fluorescent dyes. Pulses of laser light can also change the
volume of water inside cells in a way that plumps up
wrinkles and makes skin look younger, the researchers found
in an earlier study. "The potential applications of the
technique range from anticancer strategies to the design
principles of nano-steam engines," the report states. Using
the so-called Liquidrom ambient approach, developed by
Aziz's group, the researchers combined for the first time
laser irradiation with soft X-rays obtained from a cyclotron
radiation source to explore the molecular structure of
interfacial water layers under ambient conditions.
The researchers now showed that laser light aimed at a cell
causes the water inside the cell to expand. When the light
goes off, the volume of water collapses again, creating a
strong pull that also sucks in the water surrounding the
cell. This "breathing in and out" of the water molecules can
pull chemotherapy drugs into a cell faster than they would
normally penetrate, the researchers found. "In other words,
we discovered a powerful method to kill cancer cells by
pumping anti-cancer drugs into them via laser light," said
Sommer. The study was partly supported by
the Helmholtz-Gemeinschaft. The Journal of
Physical Chemistry Letters: "Breathing
Volume into Interfacial Water with Laser Light" [J.
Phys. Chem. Lett., 2011, 2 (6), pp 562–565]. |
Discovery of a biochemical basis for broccoli's
cancer-fighting ability
They found for the first time that
certain substances in the vegetables appear to target and
block a defective gene associated with cancer. Their report,
which could lead to new strategies for preventing and
treating cancer, appears in ACS' Journal of Medicinal
Chemistry.
Fung-Lung Chung and colleagues showed in
previous experiments that substances called isothiocyanates
(or ITCs) — found in broccoli, cauliflower, watercress, and
other cruciferous vegetables — appear to stop the growth of
cancer. But nobody knew exactly how these substances work, a
key to developing improved strategies for fighting cancer in
humans. The tumor suppressor gene p53 appears to play a key
role in keeping cells healthy and preventing them from
starting the abnormal growth that is a hallmark of cancer.
When mutated, p53 does not offer that protection, and those
mutations occur in half of all human cancers. ITCs might
work by targeting this gene, the report suggests.
The scientists studied the effects of
certain naturally-occurring ITCs on a variety of cancer
cells, including lung, breast and colon cancer, with and
without the defective tumor suppressor gene. They found that
ITCs are capable of removing the defective p53 protein but
apparently leave the normal one alone. Drugs based on
natural or custom-engineered ITCs could improve the
effectiveness of current cancer treatments or lead to new
strategies for treating and preventing cancer.
The authors acknowledged funding from the
Ruth L. Kirschstein National Research Service Award and a
grant from the National Cancer Institute of the National
Institutes of Health.
Journal of Medicinal Chemistry: "Selective
Depletion of Mutant p53 by Cancer Chemoprevention
Isothiocyanates and Their Structure-Activity Relationships"
[J. Med. Chem., 2011, 54 (3), pp 809–816; DOI:
10.1021/jm101199t]. |
Banana peels get a second life as water purifier
To the surprisingly inventive uses for
banana peels — which include polishing silverware, leather
shoes, and the leaves of house plants — scientists have
added purification of drinking water contaminated with
potentially toxic metals. Their report, which concludes that
minced banana peel performs better than an array of other
purification materials, appears in ACS's journal Industrial
& Engineering Chemistry Research.
Gustavo Castro and colleagues note that
mining processes, runoff from farms, and industrial wastes
can all put heavy metals, such as lead and copper, into
waterways. Heavy metals can have adverse health and
environmental effects. Current methods of removing heavy
metals from water are expensive, and some substances used in
the process are toxic themselves. Previous work has shown
that some plant wastes, such as coconut fibers and peanut
shells, can remove these potential toxins from water. In
this report, the researchers wanted to find out whether
minced banana peels could also act as water purifiers.
The researchers found that minced banana
peel could quickly remove lead and copper from river water
as well as, or better than, many other materials. A
purification apparatus made of banana peels can be used up
to 11 times without losing its metal-binding properties,
they note. The team adds that banana peels are very
attractive as water purifiers because of their low cost and
because they don't have to be chemically modified in order
to work.
The authors acknowledge funding from the
São Paulo Research Foundation.
Industrial & Engineering Chemistry
Research: "Banana
Peel Applied to the Solid Phase Extraction of Copper and
Lead from River Water: Preconcentration of Metal Ions with a
Fruit Waste" [Ind. Eng. Chem. Res., 2011, 50 (6),
pp 3446–3451]. |
An advance toward blood transfusions that require no
typing
Scientists are reporting an "important
step" toward development of a universal blood product that
would eliminate the need to "type" blood to match donor and
recipient before transfusions. A report on the
"immunocamouflage" technique, which hides blood cells from
antibodies that could trigger a potentially fatal immune
reaction that occurs when blood types do not match, appears
in the ACS journal, Biomacromolecules.
Maryam Tabrizian and colleagues note that
blood transfusions require a correct match between a donor
and the recipient's blood. This can be a tricky proposition
given that there are 29 different red blood cells types,
including the familiar ABO and Rh types. The wrong blood
type can provoke serious immune reactions that result in
organ failure or death, so scientists have long sought a way
to create an all-purpose red blood cell for transfusions
that doesn't rely on costly blood typing or donations of a
specific blood type.
To develop this "universal" red blood
cell, the scientists discovered a way to encase living,
individual red blood cells within a multilayered polymer
shell. The shell serves as a cloaking device, they found,
making the cell invisible to a person's immune system and
able to evade detection and rejection. Oxygen can still
penetrate the polymer shell, however, so the red blood cells
can carry on their main business of supplying oxygen to the
body. "The results of this study mark an important step
toward the production of universal RBCs," the study states.
The authors acknowledge funding from the
Fonds de la Recherche en Santé du Québec, the Natural
Sciences and Engineering Council of Canada, the Canadian
Institutes for Health Research and FQRNT-Centre for
Biorecognition and Biosensors.
Biomacromolecules: "Investigation
of Layer-by-Layer Assembly of Polyelectrolytes on Fully
Functional Human Red Blood Cells in Suspension for
Attenuated Immune Response" [Article ASAP; DOI:
10.1021/bm101200c]. |
New molecular robot can be programmed to follow instructions
Scientists have developed a programmable
"molecular robot" - a sub-microscopic molecular machine made
of synthetic DNA that moves between track locations
separated by 6nm. The robot, a short strand of DNA, follows
instructions programmed into a set of fuel molecules
determining its destination, for example, to turn left or
right at a junction in the track. The report, which
represents a step toward futuristic nanomachines and
nanofactories, appears in ACS's Nano Letters.
Andrew Turberfield and colleagues point
out that other scientists have developed similar DNA-based
robots, which move autonomously. Some of these use a biped
design and move by alternately attaching and detaching
themselves from anchor points along the DNA track, foot over
foot, when fuel is added. Scientists would like to program
DNA robots to autonomously walk in different directions to
move in a programmable pattern, a key to harnessing their
potential as cargo-carrying molecular machines.
The scientists describe an advance toward
this goal — a robot that can be programmed to choose among
different branches of a molecular track, rather than just
move in a straight line. The key to this specialized
movement is a so-called "fuel hairpin," a molecule that
serves as both a chemical energy source for propelling the
robot along the track and as a routing instruction. The
instructions tell the robot which point is should move to
next, allowing the selection between the left or right
branches of a junction in the track, precisely controlling
the route of the robot — which could potentially allow the
transport of pharmaceuticals or other materials.
The authors acknowledged funding from the
Engineering and Physical Sciences Research Council (EPSRC).
Nano Letters: "A
Programmable Molecular Robot" [Nano Lett., 2011, 11 (3),
pp 982–987]. |
Hair dyeing poised for first major transformation in 150 years
Technological progress may be fast-paced
in many fields, but one mundane area has been almost left in
the doldrums for the last 150 years: The basic technology
for permanently coloring hair. That's the conclusion of an
analysis of almost 500 articles and patents on the chemistry
of permanent hair dyeing, which foresees much more
innovation in the years ahead, including longer lasting,
more-natural-looking dyes and gene therapy to reverse the
gray. The article appears in ACS's journal Chemical Reviews.
Robert Christie and Olivier Morel note
that hair dye already is a multibillion dollar international
industry, poised for even greater expansion in the future
due to the graying of a global population yearning to cling
to appearances of youth. Most permanent hair coloring
technology, however, is based on a 150-year-old approach
that uses p-phenylenediamine (PPD), a chemical that produces
darker, browner shades when exposed to air. Concern over the
safety of PPD and other hair dye ingredients, and demand for
more convenient hair dyeing methods, has fostered an upswing
in research on new dyes and alternative hair coloring
technologies.
The scientists describe progress toward
those goals. Future hair coloring techniques include
nano-sized colorants, for instance. Composed of pigments
1/5,000th the width of a human hair, they will penetrate the
hair and remain trapped inside for longer-lasting hair
coloration. Scientists also are developing substances that
stimulate the genes to produce the melanin pigment that
colors hair. These substances promise to produce a wider
range of more natural-looking colors, from blond to dark
brown and black, with less likelihood of raising concerns
about toxicity and better prospects for more natural
results. Other new technologies may stop graying of the hair
or prevent its formation altogether, the scientists say.
Chemical Reviews: "Current
Trends in the Chemistry of Permanent Hair Dyeing" [Chem.
Rev., Article ASAP 2011; DOI:
10.1021/cr1000145]. |
Does fluoride really fight cavities by 'the skin of the teeth'?
In a study that the authors describe as
lending credence to the idiom, "by the skin of your teeth,"
scientists are reporting that the protective shield fluoride
forms on teeth is up to 100 times thinner than previously
believed. It raises questions about how this renowned
cavity-fighter really works and could lead to better ways of
protecting teeth from decay, the scientists suggest. Their
study appears in ACS's journal Langmuir.
Frank Müller and colleagues point out
that tooth decay is a major public health problem worldwide.
In the United States alone, consumers spend more than $50
billion each year on the treatment of cavities. The fluoride
in some toothpaste, mouthwash and municipal drinking water
is one of the most effective ways to prevent decay.
Scientists long have known that fluoride makes enamel — the
hard white substance covering the surface of teeth — more
resistant to decay. Some thought that fluoride simply
changed the main mineral in enamel, hydroxyapatite, into a
more-decay resistant material called fluorapatite.
The new research found that the
fluorapatite layer formed in this way is only 6 nanometers
thick. It would take almost 10,000 such layers to span the
width of a human hair. That's at least 10 times thinner than
previous studies indicated. The scientists question whether
a layer so thin, which is quickly worn away by ordinary
chewing, really can shield teeth from decay, or whether
fluoride has some other unrecognized effect on tooth enamel.
They are launching a new study in search of an answer.
The authors acknowledge support from
Deutsche Forschungsgemeinschaft and Saarland Ministry of
Finances.
Langmuir: "Elemental
Depth Profiling of Fluoridated Hydroxyapatite: Saving Your
Dentition by the Skin of Your Teeth?" [2010, 26 (24), pp
18750–18759; DOI: 10.1021/la102325e]. |
Polishing the apple's popular image as a healthy food
Scientists are reporting the first
evidence that consumption of a healthful antioxidant
substance in apples extends the average lifespan of test
animals, and does so by 10 percent. The new results,
obtained with fruit flies — stand-ins for humans in hundreds
of research projects each year — bolster similar findings on
apple antioxidants in other animal tests. The study appears
in ACS's Journal of Agricultural and Food Chemistry.
Zhen-Yu Chen and colleagues note that
damaging substances generated in the body, termed free
radicals, cause undesirable changes believed to be involved
in the aging process and some diseases. Substances known as
antioxidants can combat this damage. Fruits and vegetables
in the diet, especially brightly colored foods like
tomatoes, broccoli, blueberries, and apples are excellent
sources of antioxidants. A previous study with other test
animals hinted that an apple antioxidant could extend
average lifespan. In the current report, the researchers
studied whether different apple antioxidants, known as
polyphenols, could do the same thing in fruit flies.
The researchers found that apple
polyphenols not only prolonged the average lifespan of fruit
flies but helped preserve their ability to walk, climb and
move about. In addition, apple polyphenols reversed the
levels of various biochemical substances found in older
fruit flies and used as markers for age-related
deterioration and approaching death. Chen and colleagues
note that the results support those from other studies,
including one in which women who often ate apples had a
13-22 percent decrease in the risk of heart disease, and
polish the apple's popular culture image as a healthy food.
Journal of Agricultural and Food
Chemistry: "Apple
Polyphenols Extend the Mean Lifespan of Drosophila
melanogaster" [J. Agric. Food Chem., 2011, 59
(5), pp 2097–2106]. |
New treaty on search for life-saving medicines in remote
areas
Real-life scientists, whose work has
overtones of Indiana Jones as they search for plants in
remote areas of the world that could become the source of
life-saving new medicines, are currently trying to figure
out how a new international agreement on biodiversity will
affect their work. That's the topic of an article in
Chemical & Engineering News (C&EN), ACS's weekly
newsmagazine.
C&EN Associate Editor Carmen Drahl
explains that environment ministers from 200 countries
hammered out the agreement late last year. Called the Nagoya
protocol, it extends a 1993 United Nations treaty declaring
that nations have sovereign rights to the biological
materials within their territory. Those materials - which
include plants, microbes, and other living things - have
been a rich source of so-called "natural products." Almost
70 percent of today's medicines are either natural products
or are derived from natural products.
The new treaty clarifies what agencies
scientists who collect plant and other materials should
approach for official clearance. It also requires countries
that ratify the agreement to establish a "national focal
point," such as a university, government agency, or other
contracting institution, for making such decisions. In
addition, biodiversity-rich nations would receive
compensation for medicines and other items commercialized
from natural products discovered in their country. Many
natural product hunters are grateful for the clarity the
treaty provides. But some worry that it could also trigger
new regulations that could delay or stifle their searches.
Chemical and Engineering News: "Navigating
Nagoya" [Volume 89, Number 9, DOI
10.1021/CEN022211145306]. |
Blood protein in lung cancer could improve diagnosis and treatment
Scientists are reporting discovery of a
protein in the blood of lung cancer patients that could be
used in a test for the disease — difficult to diagnose in
its earliest and most treatable stages — and to develop
drugs that stop lung cancer from spreading. Their study
appears in ACS's Journal of Proteome Research.
In the report, Je-Yoel Cho and colleagues
note that lung cancer is the leading cause of cancer deaths
worldwide. Lung cancer is so deadly because of its tendency
to spread — or metastasize — to distant sites in the body,
such as the liver or the brain. Early detection could
improve survival rates, but it is very difficult to detect
lung cancer at early stages with today's technology. To find
a better diagnostic tool, the researchers studied the
proteins in the blood of lung cancer patients in search of
red flags that could signal the disease's presence. They
focused on adenocarcinoma, which accounts for 1 in 3 cases
and is the most rapidly increasing form of lung cancer in
women.
Cho and colleagues found elevated levels
of a protein called serum amyloid A (SAA) in the blood and
lung tissue of lung adenocarcinoma patients, compared to
healthy people. Their work showed that high amounts of SAA
were unique to lung cancers (compared with other lung
diseases or other cancers) and that the protein was involved
in metastasis of cancer cells from the original tumor site.
The researchers say that the protein could be used as a
diagnostic marker for lung cancer and as a target for
developing drugs that stop metastasis.
The authors acknowledge funding from the
Korean Ministry of Education, Science and Technology; the
Small and Medium Business Administration; and the Ministry
of Knowledge Economy.
Journal of Proteome Research: "Identification
and Validation of SAA as a Potential Lung Cancer Biomarker
and its Involvement in Metastatic Pathogenesis of Lung
Cancer" [J. Proteome Res., Article ASAP 2010;
DOI: 10.1021/pr101154j]. |
|
|



