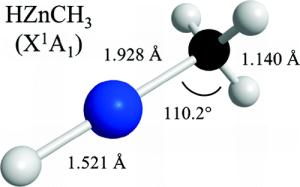
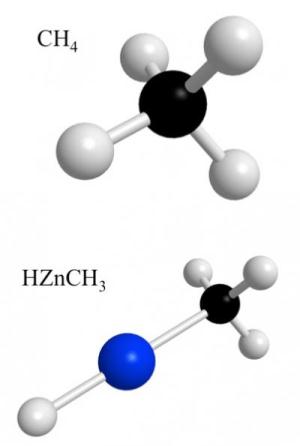

For the first time, chemists have succeeded in plugging a metal atom into a methane gas molecule, thereby creating a new compound that could be a key in opening up new production processes for the chemical industry, especially for the synthesis of organic compounds, which in turn might have implications for drug development.
The UA research group also is the first to determine the precise structure of this "metal-methane hybrid" molecule, predicted by theoretical calculations but until now never observed in the real world.
The discovery is published in the Journal of the American Chemical Society and was selected for a news spotlight in Chemical and Engineering News magazine, the weekly publication of the American Chemical Society, because of its significance.
In the chemistry world, seemingly simple actions can have big implications. For example, squeezing zinc atoms into methane gas molecules. This so-called metal-methane insertion is neither a complicated chemical reaction nor something that is likely to happen in nature, but it's very hard to do in the lab. What is even harder is figuring out what the resulting molecule looks like. But chemists like to tinker with things. And the chemical industry likes to tinker with things even more, especially when that tinkering could lead to useful products.
"There is a big push in the chemical industry and in chemistry in general, to make use of fairly common organic compounds such as methane and turn them into something that can serve as a source for a product," said Lucy Ziurys, who led the research effort. "For example, a plastic or a polymer, something that is more useful than just taking the methane and burning it."
"Our finding could make industrial applications easier, cheaper, quicker, and they could start with this simple compound, methane. They could convert it to all kinds of more complex and more valuable products."
"Gaining a better understanding of these simple reactions that we really don't understand at the basic level always has applications to more complicated systems," Ziurys added.
Ziurys is a professor of chemistry and a professor of astronomy with joint appointments in the UA's department of chemistry and biochemistry and Steward Observatory.
Methane gas, produced naturally by decaying organic matter, is familiar to many as the main ingredient in natural gas. It is also a potent greenhouse gas, more powerful than carbon dioxide. Budding chemistry students are introduced to methane as the simplest of all organic molecules. All organic molecules contain carbon and hydrogen in one way or another, which sets them apart from inorganic molecules such as table salt, which contains sodium and chloride, but no carbon or hydrogen.
When it comes to interacting with other molecules, methane is a bit anti-social. Or, as chemists put it, it is "inert," meaning one has to do a whole lot of nudging and prodding to get methane to bond with other chemicals. Chemists call this nudging and prodding "activating." And that is precisely what the tinkerers in the chemical science community and the industry would like to be able to do.
Said Ziurys: "One way to get these molecules more reactive is by what is called metal insertion. The metal inserts itself into the methane molecule and thereby activates it. It makes it more prone to reacting with something else. So you could then take this activated methane and make, say, methanol."
"Until now, there was no complete evidence that the metal actually inserts itself into the molecule bond and forms this complex. People just assumed it did," she said. "But we are the first to actually prove the existence of the complex and describe its structure to a very high degree of accuracy. It's the first time anyone has been able to do this."
The new compound is stable for a few seconds - long enough for industrial applications to immediately convert it to something else.
To create the molecule and analyze its structure, Ziurys' research group heated zinc until it vaporized in a vacuum chamber and added methane gas. An electrical discharge fed energy into the system, converting the gas mixture into glowing plasma, sparking the formation of the metal-methane molecule. Most of the experiments were done by Michael Flory, a former graduate student of Ziurys', as part of his doctoral thesis.
"We made the molecule in a gas phase, which is the only way we can really obtain a good measurement of the structure," Ziurys said. "Almost every theory paper said this couldn't be made in the gas phase, which is probably why nobody really tried it before."
"Our data show that zinc goes right in and pops into that bond that links the carbon atom to one of the four hydrogen atoms in methane. People have speculated on that, but this is the first time anyone has shown that that is what actually happens."
Because none of these processes are visible to the naked eye, the scientists used a microwave source to send electromagnetic energy at defined wavelengths through the plasma. Here is the trick: Any given molecule absorbs some of that energy at a very distinct wavelength, depending on its chemical structure. By detecting those dips in the energy inside the chamber, each species of molecule leaves its own energy dip as a telltale signature that can be picked up by a detector. This process is called direct-absorption spectroscopy.
As is often the case with scientific discoveries, Ziurys' team was after a completely different type of molecule.
"We searched in our spectra for them, but we never found them. Instead, we found our methane with zinc in it," Ziurys said. "That really surprised us. We didn't expect that to be there."
The group did the necessary experiments to confirm the structure of the elusive molecule and everything fell together, Ziurys said.
"We knew exactly what we had. Those molecules are floating around and they rotate, generating a certain spectral pattern, depending on the mass of the molecule and the bond lengths between the atoms they are made of. We then exchange the atoms for slightly different versions with different masses, and we get a slight change in inertia, which results in a changed rotational pattern. Then we apply the math and we get a structure."
Nature makes abundant use of metal atoms embedded in complex organic molecules. In fact, metals are involved in almost any sort of complicated chemical reaction in living systems. One example is hemoglobin and iron, the large protein molecule that contains iron atoms in precise arrangements to capture oxygen and transport it around in our blood stream.
"We know that metals play important roles in biology, but we don't have a very good understanding of those processes," said Ziurys. "If we did, we'd be able to use them much better."
According to Ziurys, zinc is one of the most biologically important metals, used by many enzymes to perform their jobs.
"How does Zinc react? How does it work? If we understand how it does in simple molecules like methane, eventually we should be able to generalize to much more complicated systems like enzymes."
Further Information:
Michael A. Flory, Aldo J. Apponi, Lindsay N. Zack, and Lucy M. Ziurys:
Activation of Methane by Zinc: Gas-Phase Synthesis, Structure, and Bonding of HZnCH3.
In: Journal of the American Chemical Society; 132 (48), pp 17186 - 17192, November 15, 2010, DOI 10.1021/ja106121v
Source: University of Arizona, USA
Last update: 30.12.2010
Perma link: https://www.internetchemistry.com/news/2010/dec10/methylzinc-hydride-molecule.php
More chemistry: index | chemicals | lab equipment | job vacancies | sitemap
Internetchemistry: home | about | contact | imprint | privacy
© 1996 - 2023 Internetchemistry
