
Because they can remove carbon dioxide from the flue gases of coal-burning facilities such as power plants, solid materials containing amines are being extensively studied as part of potential CO2 sequestration programs designed to reduce the impact of the greenhouse gas.
But although these adsorbent materials do a good job of trapping the carbon dioxide, commonly-used techniques for separating the CO2 from the amine materials - thereby regenerating them for re-use - seem unlikely to be suitable for high-volume industrial applications.
Now, researchers have demonstrated a relatively simple regeneration technique that could utilize waste steam generated by many facilities that burn fossil fuels. This steam-stripping technique could produce concentrated carbon dioxide ready for sequestration in the ocean or deep-earth locations - while readying the amine materials for further use.
"We have demonstrated an approach to developing a practical adsorption process for capturing carbon dioxide and then releasing it in a form suitable for sequestration," said Christopher Jones, a professor in the School of Chemical & Biomolecular Engineering at the Georgia Institute of Technology.
The research was reported online June 23, 2010 in the early view version of the journal ChemSusChem. The work was supported by New York-based Global Thermostat, LLC., a company that is developing and commercializing technology for the direct capture of carbon dioxide from the air.
Amine sorbents are often regenerated through a process that involves a change in temperature to supply the energy required to break the amine-carbon dioxide chemical bonds.
For convenience, researchers commonly remove the CO2 by heating the amine material in the presence of a flowing gas such as nitrogen or helium. That removes the carbon dioxide, but mixes it with the flowing gas - regenerating the material, but leaving the CO2 mixed with nitrogen or helium.
Another approach is to heat the material in a carbon dioxide stream, but that is less efficient and can lead to fouling of the amine.
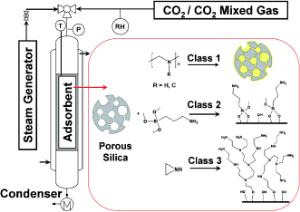
Jones and his team from Georgia Tech, SRI International and Global Thermostat took a different approach, heating the sorbent amine in steam at a temperature of approximately 105 degrees Celsius, causing the carbon dioxide to separate from the material. The steam can then be compressed, condensing the water and leaving a concentrated flow of carbon dioxide suitable for sequestration or other use - such as a nutrient for algae growth.
Because most coal-burning facilities generate steam, some of that might be bled off to achieve the separation and regeneration without a significant energy penalty. "In many facilities, steam at this temperature would have no other application, so using it for this purpose would not have a significant cost to the plant," Jones noted.
The researchers studied three common formulations of the amine material:
Class 1 adsorbents based on porous supports impregnated with monomeric or polymeric amines,
Class 2 adsorbents that are covalently linked to a solid support, and
Class 3 adsorbents based on porous supports upon which aminopolymers are polymerized in-situ, starting from an amine-containing monomer.
The adsorbents were studied through three cycles of carbon dioxide adsorption and steam-stripping. The researchers found differences in how each material was affected by the steam-stripping; performance of the most stable material actually improved, while the least stable material suffered a 13 percent efficiency decline.
"Steam-stripping is widely used in other separation processes, but has never been reported for use with supported amine materials, perhaps due to concerns about sorbent stability," Jones said. "We reported three uses of the materials in the paper and have only tested them through five or six uses, but we expect the materials could be used many more times. To be practical, the amine-containing materials need to be useful through thousands of cycles."
Pilot-scale carbon dioxide separation facilities are already in operation using amines dissolved in water. Because of the energy required to regenerate the liquid solutions, many researchers have been examining solid amines - but the work so far has focused mostly on improving the efficiency of the materials, he added.
Though much remains to be done before solid amine materials can be used in large-scale applications, Jones believes the study demonstrates that improved materials can be developed with properties tailored for the steam regeneration process.
"We believe there is potential for development of materials that will be stable for long-term use during regeneration using this technique," he said. "This study lays the groundwork for an array of future studies that will lead to an understanding of the structural changes induced by steam-stripping."
Further Information:
Wen Li, Sunho Choi, Jeffery H. Drese, Marc Hornbostel, Gopala Krishnan, Peter M. Eisenberger, Christopher W. Jones:
Steam-Stripping for Regeneration of Supported Amine-Based CO2 Adsorbents.
In: ChemSusChem; published online: 23 Jun 2010, DOI 10.1002/cssc.201000131
Source: Georgia Institute of Technology, USA
Last update: 21.07.2010
Perma link: https://www.internetchemistry.com/news/2010/jul10/amine-based-carbon-sequestration.php
More chemistry: index | chemicals | lab equipment | job vacancies | sitemap
Internetchemistry: home | about | contact | imprint | privacy
© 1996 - 2023 Internetchemistry
