Since their discovery in the mid 1980s, fullerenes have caused a sensation. The tiny hollow spheres made of 60 carbon atoms, constructed out of pentagons and hexagons like miniature soccer balls, have unusual physical properties. In the meantime, a variety of fullerene-containing materials have been developed. Now a new variant has been made: A Russian and Japanese team has produced the first material made of two-dimensional fullerene layers that acts like a metal.
As the researchers report in the journal Angewandte Chemie, this new class of compounds could open a route toward novel superconducting materials.
All previous fullerene-containing crystals with metallic properties have been one- or three-dimensional structures and contained metal elements. Dmitri V. Konarev, Gunzi Saito, and their co-workers from Chernogolovka, Kyoto, and Nagoya had the ambition to make a metallic conducting fullerene "salt" containing two-dimensional fullerene layers. In addition, they wanted it to be free of metal ions, containing only the elements carbon, hydrogen, and nitrogen.
For this to work, three different components were needed:
1) fullerene anions, negatively charged "miniature soccer balls";
2) positively charged organic counterions (cations); and
3) large neutral organic molecules.
Component 2, the cations, are needed to maintain the right distribution of electrical charge within the material. The neutral compound 3 assures the correct spatial arrangement of the individual building blocks within the crystal structure.
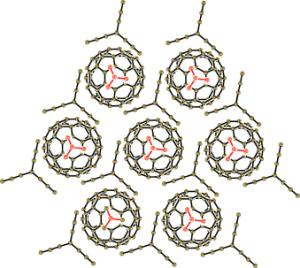
The problem: fullerene anions in a crystal have a tendency to form pairs. In order for the material to behave as a metal, the fullerene anions need to be densely packed within their layer. Only when the geometry and size of the neutral partner are exactly right does this work. The team chose to use triptycene as the neutral component; this is an aromatic ring system whose shape is reminiscent of a three-bladed propeller. The organic cation they used has a cage-like structure.
The result is a crystal in which fullerene layers alternate with layers made of the two other partners. The fullerene layer has a honeycomb structure in which every tiny, negatively charged "soccer ball" has six adjacent neighbors. The fullerene layers are highly conducting like a metal - even down to temperatures near absolute zero (1.9 K), which is very unusual.
It should be possible to produce other materials in this class by varying the individual partners. The researches expect that this will produce materials with exotic electronic properties, such as novel superconductors or spin liquids, which are materials that show an unusual magnetic state at absolute zero.
Further Information:
Dmitri V. Konarev, Salavat S. Khasanov, Akihiro Otsuka, Mitsuhiko Maesato, Prof. Gunzi Saito, Prof. Rimma N. Lyubovskaya:
A Two-Dimensional Organic Metal Based on Fullerene.
In: Angewandte Chemie International Edition; Volume 49 Issue 28, Pages 4829 - 4832, published online: 8 Jun 2010, DOI 10.1002/anie.201001463
Source: Angewandte Chemie International Edition, press release 20/2010
Last update: 05.07.2010
Perma link: https://www.internetchemistry.com/news/2010/jul10/organic-metal-made-fullerenes.php
More chemistry: index | chemicals | lab equipment | job vacancies | sitemap
Internetchemistry: home | about | contact | imprint | privacy
© 1996 - 2023 Internetchemistry
