In the nanoworld many things are different. Scientists only recently started unveiling and harnessing the underlying laws and principles. A team associated with Professor Johannes Barth from the Physics Department of the TU München have now succeeded in capturing rod-shaped molecules in a two-dimensional network in such a way that they autonomously form small rotors that turn in their honeycomb-like cages.
Nature itself provides the role model for such self-organizing systems. This is how proteins bring reactants so close together that reactions can take place - reactions that are possible only in very close proximity. These effects are put to use in catalysts: surface reactants find their way to each other on the surface of these facilitators. However, the coveted dream of using self-organization effects in such a way that nano machines assemble themselves is still a thing of the future.
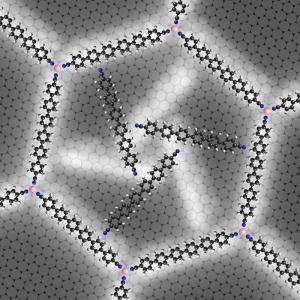
The rotors developed in Garching are an important step in this direction. First, the physicists built up an extensive nano lattice by allowing cobalt atoms and rod-shaped molecules of sexiphenyl-dicarbonitrile to react with each other on a silver surface. This results in a honeycomb-like lattice of extreme regularity with astonishing stability. Just like graphene, for which its discoverers were awarded the Nobel Prize only a few weeks ago, this lattice is exactly one atom thick.
When the researchers added further molecular building blocks, the rods spontaneously gathered, typically in groups of three, in a honeycomb cell while neighboring cells remained empty. The chummy molecules must have had a reason for organizing themselves in threesomes. Under a scanning tunneling microscope the scientists were able to recognize why. The three molecules oriented themselves in such a way that the nitrogen ends each faced a phenyl-ring hydrogen atom. This triple-bladed rotor arrangement is so energetically advantageous that the molecules maintain this structure even when thermal energy drives it to rotation.
Because the honeycomb-cell is not round, but hexagonal, there are two different positions for the rotors that can be distinguished as a result of the interactions between the outer nitrogen atoms and the hydrogen atoms of the cell wall. Furthermore, the three molecules arrange in a clockwise and a counter-clockwise manner. In experiments at various carefully controlled temperatures the physicists were able to "freeze" all four states and examine them closely. They could thus determine the energy of these thresholds from the temperature at which the rotation resumed.
"We hope that in future we will be able to extend these simple mechanical models to optical or electronic switching," says Professor Johannes Barth. "We can set a specific cell size, we can specifically bring in further molecules and study their interaction with the surface and the cell wall. These self-organizing structures hold enormous potential."
The research was funded by the European Union (ERC Advanced Grant MolArt), as well as from the Institute for Advanced Study (TUM-IAS), the International Graduate School for Science and Engineering (IGSSE) and the Catalysis Research Center (CRC) at the TU München. The publication resulted from the collaboration with scientists at the Institute of Nanotechnology of the Karlsruhe Institute of Technology and the Institute of Material Physics and Chemistry of the University of Strasbourg.
Further Information:
Dirk Kühne, Florian Klappenberger, Wolfgang Krenner, Svetlana Klyatskaya, Mario Ruben, Johannes V. Barth:
Rotational and constitutional dynamics of caged supramolecules.
In: Proceedings of the National Academy of Sciences; PNAS, published online before print November 22, 2010, DOI 10.1073/pnas.1008991107
Source: Technical University of Munich, TUM, Germany
Last update: 25.11.2010
Perma link: https://www.internetchemistry.com/news/2010/nov10/self-assembly-of-nano-rotors.php
More chemistry: index | chemicals | lab equipment | job vacancies | sitemap
Internetchemistry: home | about | contact | imprint | privacy
© 1996 - 2023 Internetchemistry
