UPTON, NY - A study published as the "Paper of the Week" in the Journal of Biological Chemistry details how zinc, an element fundamental to cell growth, enters the cell via zinc-specific uptake proteins.
The research, conducted at the U.S. Department of Energy's (DOE) Brookhaven National Laboratory, is the first to purify this kind of protein and study its role in zinc uptake.
Zinc is crucial to the health of all living organisms. At the cellular level, zinc is responsible for cell growth, which in turn affects the health, growth, and reproduction of an organism.
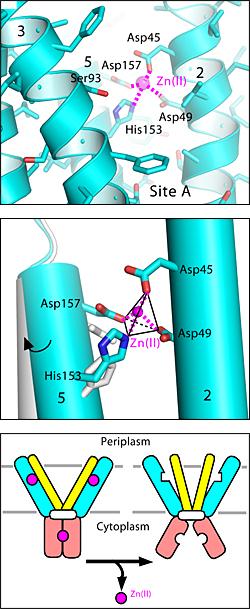
While there are six classes of known proteins that act as transporters or channels enabling zinc to cross the cell membrane, scientists have identified one metal-specific family of proteins whose purpose is to facilitate the cell's zinc uptake. These are called ZIP proteins, a reference to their resemblance to zinc-regulated and ion-regulated transporter proteins.
The exact mechanism by which ZIP proteins facilitate zinc uptake has been a mystery. Brookhaven scientists led by biologist Dax Fu - who have characterized other zinc-specific proteins that maintain healthy levels of zinc on either side of the cell membrane - have now taken a closer look at this process.
A long-held belief has posited that ZIP proteins work like elevators, pumping zinc across the cell membrane and into the cell. However, Fu and his colleagues found no evidence to support this explanation.
"This was a big surprise. For the last fifteen years, the assumption has been that the ZIP protein acts like a pump or elevator," said Fu. "Instead, we have found that ZIP is more like a door."
Fu and his colleagues have studied a ZIP protein provided by the New York Consortium on Membrane Protein Structure and derived from the bacteria Bordatella bronchiseptica. They expressed the protein in Eschericia coli, a bacteria whose zinc regulation has been well documented. Brookhaven's Jin Chai purified and concentrated the samples before exposing them to zinc. By reconstituting the purified ZIP proteins in this controlled manner, the scientists ensured that their sample would reflect only the ZIP mechanism for cellular zinc uptake.
"It's important to note that people have been trying to purify these proteins for a long time. Purification of the first ZIP family member opens the door for detailed structural and functional analysis at the molecular level," Fu said.
Using a fluorescent indicator, Brookhaven's Wei Lin then conducted several measurements to characterize zinc uptake, with attention to changes in zinc concentration, temperature, acidity, and electric charge.
The Brookhaven team found evidence of electrodiffusion. Ions diffuse by moving from a region with a high concentration to one of a lower concentration - like diners who relocate from a crowded dining hall to an adjoining, empty coffee room. In electrodiffusion, the diffused ions also change the electric charge of the space that they occupy. The imbalance in charge created by zinc ions moving into the cell builds during zinc uptake and acts against the concentration gradient, eventually causing zinc uptake to stop.
Based in part on the studies of similar metal-specific proteins, Fu and his colleagues have postulated that the ZIP protein allows zinc ion diffusion by providing an opening that is specifically shaped for zinc coordination chemistry. This hypothesis will eventually be confirmed in studies that crystallize and examine the ZIP protein at the atomic level.
"We are driven by our curiosity - we want to know how this works," Fu said.
Aside from satisfying scientific curiosity, this understanding could have a big impact. Zinc uptake at the cellular level is implicated in a range of biomedical and energy research. For example, in green plants, carbonic acid is converted to CO2 in a chemical reaction that is catalyzed by a zinc enzyme.
Zinc deficiency, therefore, has a direct impact on the carbohydrate metabolism of plants. For researchers developing biofuels energy sources, these systems and the role of zinc transporters in the conversion of energy into carbohydrates, are important objects of study. Developing a better understanding of zinc uptake can provide greater insight into these processes and will inform future discoveries.
In addition to the Brookhaven researchers, James Love of the New York Structure Biology Center contributed to this research. Funding and support for the work came from the DOE Office of Science and from the National Institutes of Health.
Further Information:
Wei Lin, Jin Chai, James Love and Dax Fu:
Selective electrodiffusion of zinc ions in a Zrt-, Irt-like protein, ZIPB.
In: Journal of Biological Chemistry; published on September 28, 2010, DOI 10.1074/jbc.M110.180620
Source: DOE/Brookhaven National Laboratory, BNL, USA
Last update: 25.11.2010
Perma link: https://www.internetchemistry.com/news/2010/nov10/zip-protein-act-as-doorway-for-zinc-to-the-cell.php
More chemistry: index | chemicals | lab equipment | job vacancies | sitemap
Internetchemistry: home | about | contact | imprint | privacy
© 1996 - 2023 Internetchemistry
