
Noble metals such as platinum and palladium are becoming increasingly important because of growth in environmentally friendly applications such as fuel cells and pollution control catalysts. But the world has limited quantities of these materials, meaning manufacturers will have to rely on efficient recycling processes to help meet the demand.
Existing recycling processes use a combination of two inorganic acids known as "aqua regia" to dissolve noble metals, a class of materials that includes platinum, palladium, gold and silver. But because the metals are often dissolved together, impurities introduced in the recycling process may harm the efficiency of catalysts produced from the recycled materials.
Now, researchers at the Georgia Institute of Technology have developed a new organic solvent process that may help address the problem - and open up new possibilities for using these metals in cancer therapeutics, microelectronics and other applications.
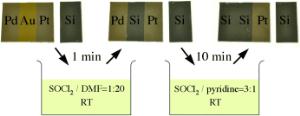
The new Georgia Tech solvent system uses a combination of two chemicals - thionyl chloride and a variety of organic reagents such as pyridine, N,N-dimethylformamide (DMF), pyrimidine or imidazole. The concentrations can be adjusted to preferentially dissolve gold or palladium, and more importantly, no combination of the organic chemicals dissolves platinum. This ability to preferentially dissolve noble metals creates a customized system that provides a high level of control over the process.
"We need to be able to selectively dissolve these noble metals to ensure their purity in a variety of important applications," said C.P. Wong, a Regents professor in the Georgia Tech School of Materials Science and Engineering. "Though we don't fully understand how it works yet, we believe this system opens a lot of new possibilities for using these metals."
A paper describing the research was published in the journal Angewandte Chemie [see below].
Catalyst systems that make use of more than one metal, such as palladium with a gold core, are becoming more widely used in industrial processes. To recycle those, the new solvent system - dubbed "organic aqua regia" - could first use a combination of thionyl chloride and DMF to dissolve out the gold, leaving hollow palladium spheres. Then the palladium spheres could be dissolved using a different combination.
So far, the researchers have demonstrated that the solvent system can selectively dissolve gold and palladium from a mixture of gold, palladium and platinum. They have also used it to remove gold from a mixture of gold and palladium.
Beyond recycling, the new solvent system could also provide new ways of producing nanometer-scale cancer chemotherapy agents that involve these metals. And the new solvent approach could have important implications for the electronics industry, which uses noble metals that must often be removed after specific processing steps. Beyond selectivity, the new approach also offers other advantages for electronics manufacturing - no potentially harmful contamination is left behind and processing is done under mild conditions.
"In semiconductor production, people want to avoid having a metal catalyst remaining in devices, but in many cases, they cannot use existing water-based processes because these can damage the semiconductor oxides and introduce contamination with free ions in the aqueous solution," explained Wei Lin, a graduate research assistant in Wong's laboratory. "Use of this organic system avoids the problem of moisture."
Use of the selective process could also facilitate recycling of noble metals used in electronics manufacturing. Wire-bonding, metallization and interconnect processes currently use noble metals.
Noble metals are also the foundation for widely-used chemotherapy agents, but the chemistry of synthesizing them involves a complex process of surfactants and precursors. Wong believes the new Georgia Tech solvent process may allow creation of novel compounds that could offer improved therapeutic effects.
"We hope this will open up some new ways of making these important pharmaceutical compounds as well as novel gold and palladium catalytic systems," he said.
Lin discovered the new solvent system by accident in 2007 while using thionyl chloride in an unrelated project that involved bonding carbon nanotubes to a gold substrate. "I left my sample in the solution and went to lunch," he recalled. "Then I received a couple of phone calls and the sample stayed in the solution for too long. When I got it out, the gold was gone."
The researchers were intrigued by the discovery and pursued an explanation as they had time over the past three years. They tested other reagents mixed with the thionyl chloride, and learned the proportions necessary for selective dissolution of palladium and gold. They worked with other researchers at Georgia Tech, including nanotechnology pioneer Zhong Lin Wang, to develop a fundamental understanding of the process - research that is continuing.
The chemicals used by the Georgia Tech research team are well known in organic chemistry, and are used today in polymer synthesis. Beyond their selectivity, the new solvent system is more environmentally friendly than traditional aqua regia - which is a combination of concentrated nitric and hydrochloric acids - and can operate at mild conditions. Potential disadvantages compared to traditional aqua regia include higher costs and slower dissolution rates.
"We have opened up a new approach to noble metals using organic chemistry," Wong added. "We don't yet thoroughly understand the mechanism by which this works, but we hope to develop a more complete understanding that may lead to additional applications."
Further Information:
Wei Lin, Rong-Wei Zhang, Seung-Soon Jang, Prof. Ching-Ping Wong, Jung-Il Hong:
Organic Aqua Regia - Powerful Liquids for Dissolving Noble Metals.
In: Angewandte Chemie International Edition; first published online: 17 September 2010, DOI 10.1002/anie.201001244
Source: Georgia Institute of Technology, USA
Last update: 31.10.2010
Perma link: https://www.internetchemistry.com/news/2010/oct10/organic-aqua-regia.php
More chemistry: index | chemicals | lab equipment | job vacancies | sitemap
Internetchemistry: home | about | contact | imprint | privacy
© 1996 - 2023 Internetchemistry
