EVANSTON, Ill. - Sugar, salt, alcohol and a little serendipity led a Northwestern University research team to discover a new class of nanostructures that could be used for gas storage and food and medical technologies. And the compounds are edible.
The porous crystals are the first known all-natural metal-organic frameworks (MOFs) that are simple to make. Most other MOFs are made from petroleum-based ingredients, but the Northwestern MOFs you can pop into your mouth and eat, and the researchers have.
"They taste kind of bitter, like a Saltine cracker, starchy and bland," said Ronald A. Smaldone, a postdoctoral fellow at Northwestern. "But the beauty is that all the starting materials are nontoxic, biorenewable and widely available, offering a green approach to storing hydrogen to power vehicles."
Smaldone is co-first author of a paper about the edible MOFs published by Angewandte Chemie. The study is slated to appear on the cover of one of the journal's November 2010 issues.
"With our accidental discovery, chemistry in the kitchen has taken on a whole new meaning," said Sir Fraser Stoddart, Board of Trustees Professor of Chemistry in the Weinberg College of Arts and Sciences at Northwestern. The implications of what Sir Fraser refers to as "Bob's your uncle chemistry" go all the way from cleaner air to healthier living, and it all comes from a product that can be washed down the sink.
Stoddart led the research group that included a trio of postdoctoral fellows in chemistry at Northwestern and colleagues from the University of California, Los Angeles (UCLA) and the University of St. Andrews in the U.K.
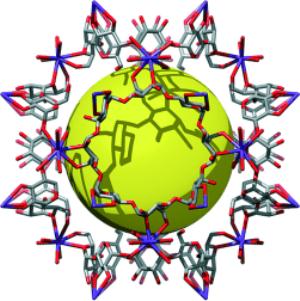
Metal-organic frameworks are well-ordered, lattice-like crystals. The nodes of the lattices are metals (such as copper, zinc, nickel or cobalt), and organic molecules connect the nodes. Within their very roomy pores, MOFs can effectively store gases such as hydrogen or carbon dioxide, making the nanostructures of special interest to engineers as well as scientists.
"Using natural products as building blocks provides a new direction for an old technology," said Jeremiah J. Gassensmith, a postdoctoral fellow in Stoddart's lab and an author of the paper.
"The metal-organic framework technology has been around since 1999 and relies on chemicals that come from crude oil," explained Ross S. Forgan, also a postdoctoral fellow in Stoddart's lab and co-first author of the paper. "Our main constituent is a starch molecule that is a leftover from corn production."
For their edible MOFs, the researchers use not ordinary table sugar but gamma-cyclodextrin, an eight-membered sugar ring produced from biorenewable cornstarch. The salts can be potassium chloride, a common salt substitute, or potassium benzoate, a commercial food preservative, and the alcohol is the grain spirit Everclear.
 |
| This simple recipe can be followed to make all-natural metal-organic frameworks. |
With these ingredients in hand, the researchers actually had set out to make new molecular architectures based on gamma-cyclodextrin. Their work produced crystals. Upon examining the crystals' structures using X-rays, the researchers were surprised to discover they had created metal-organic frameworks - not an easy feat using natural products.
"Symmetry is very important in metal-organic frameworks," Stoddart said. "The problem is that natural building blocks are generally not symmetrical, which seems to prevent them from crystallizing as highly ordered, porous frameworks."
It turns out gamma-cyclodextrin solves the problem: it comprises eight asymmetrical glucose residues arranged in a ring, which is itself symmetrical. The gamma-cyclodextrin and potassium salt are dissolved in water and then crystallized by vapor diffusion with alcohol.
The resulting arrangement - crystals consisting of cubes made from six gamma-cyclodextrin molecules linked in three-dimensions by potassium ions - was previously unknown. The research team believes this strategy of marrying symmetry with asymmetry will carry over to other materials.
The cubes form a porous framework with easily accessible pores, perfect for capturing gases and small molecules. The pore volume encompasses 54 percent of the solid body.
"We achieved this level of porosity quickly and using simple ingredients," Smaldone said. "Creating metal-organic frameworks using petroleum-based materials, on the other hand, can be expensive and very time consuming."
Stoddart added, "It is both uplifting and humbling to come to terms with the fact that a piece of serendipity could have far-reaching consequences for energy storage and environmental remediation on the one hand and food quality control and health care on the other."
The National Science Foundation and the Engineering and Physical Sciences Research Council (U.K.) supported the research.
The title of the paper is "Metal-Organic Frameworks from Edible Natural Products." In addition to Stoddart, Smaldone, Forgan and Gassensmith, other authors of the paper are Hiroyasu Furukawa and Omar M. Yaghi, from UCLA, and Alexandra M. Z. Slawin, from the University of St. Andrews.
[Article by Megan Fellman]
Further Information:
Dr. Ronald A. Smaldone, Dr. Ross S. Forgan, Dr. Hiroyasu Furukawa, Dr. Jeremiah J. Gassensmith, Prof. Alexandra M. Z. Slawin, Prof. Omar M. Yaghi, Prof. J. Fraser Stoddart:
Metal-Organic Frameworks from Edible Natural Products.
In: Angewandte Chemie International Edition; first published online: 16 August 2010, DOI 10.1002/anie.201002343
Source: Northwestern University, USA
Last update: 02.09.2010
Perma link: https://www.internetchemistry.com/news/2010/sep10/nanoporous-mof.php
More chemistry: index | chemicals | lab equipment | job vacancies | sitemap
Internetchemistry: home | about | contact | imprint | privacy
© 1996 - 2023 Internetchemistry
