 |
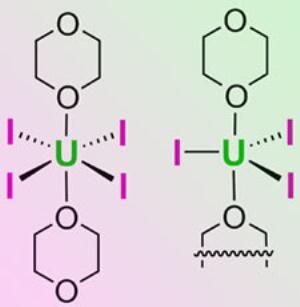
This illustration shows the structures of UI4(1,4-dioxane)2 (left) and the UI3(1,4-dioxane)1.5 complexes.
The 1,4-dioxane derivatives of uranium iodides are easy to make and can be converted into a range of other compounds.
[Credit: Jaqueline Kiplinger, LANL] |
But uranium starting materials have traditionally been relatively difficult or hazardous to produce. Now researchers at Los Alamos National Laboratory have developed a method to produce uranium starting materials in a much more benign fashion. The method, recently published in the scientific journal Organometallics, relies on a room-temperature process that reacts uranium metal in a solution of 1,4-dioxane - a liquid organic solvent - and iodine.
Conventional methods of producing uranium starting materials can require toxic chlorine-containing compounds and high temperatures or mercury iodide and low temperatures. Some of these syntheses are dangerous and generate a fair amount of waste.
"A major barrier to widespread uranium chemistry research has been access to these starting materials," said Jaqueline Kiplinger, lead scientist on the research. "Easy access to uranium(III) and -(IV) precursors can change the way people do uranium work because there is less waste, and it’s simpler, cleaner, safer, and faster."
The synthesis involves placing readily available metal uranium shavings in a liquid bath of 1,4-dioxane and iodine at room temperature and stirring. The result is either UI3(1,4-dioxane)1.5 or UI4(1,4-dioxane)2, both called uranium iodides. Both waste little of the original uranium and are highly resistant to degradation. Further, these starting materials have been used to make many other uranium compounds that are valuable in uranium research.
"It's my belief that these developments will open doors to a variety of new uranium research areas," said Kiplinger.
In a recent edition of the magazine Chemistry World, Stephen Liddle, a uranium chemistry researcher in the United Kingdom, agreed. "Historically this area has lagged behind many others, and one reason is the lack of suitable precursor materials," he said. "Hopefully these alternative uranium halides will help open up the area in general by leading to new compounds."
The research team includes Marisa Monreal, a Seaborg Graduate Student Fellow at Los Alamos, Robert Thomson and Nicholas Travia, both Seaborg Postdoctoral Fellows at Los Alamos, Thibault Cantat, Brian Scott and Jaqueline Kiplinger (all of materials physics & applications division). The research was supported by the DOE Office of Science-Heavy Element Chemistry program, the Los Alamos Laboratory Directed Research and Development program, and through Los Alamos National Laboratory Director's and G.T. Seaborg Institute for Transactinium Science Postdoctoral Fellowships.
Los Alamos enhances national security by ensuring the safety and reliability of the U.S. nuclear stockpile, developing technologies to reduce threats from weapons of mass destruction, and solving problems related to energy, environment, infrastructure, health, and global security concerns.



