Anyone who thinks amalgams are limited to tooth fillings is missing something: Amalgams, which are alloys of mercury and other metals, have been used for over 2500 years in the production of jewelry and for the extraction of metals like silver and gold in mining operations.
These days, the inverse process is of greater interest: the removal of mercury from wastewater by amalgamation with precious metals in the form of nanoparticles.
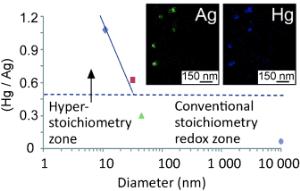
Kseniia Katok and colleagues have now reported new insights in the journal Angewandte Chemie [see below]: if the diameter of silver nanoparticles is made even smaller, significantly more mercury can be extracted relative to the amount of silver used.
In the conventional process, two silver atoms react with one mercury ion, which carries a twofold positive charge, to produce two silver ions, which go into solution, and a neutral mercury atom, which is taken up by the metallic silver particles. The stoichiometric ratio of mercury to silver is thus 1:2.
The researchers at the University of Brighton (UK) and colleagues in Kazakhstan, France and Japan have now determined that the stoichiometry of the reaction changes if the diameter of the silver nanoparticles drops below a critical 32 nm. This effect, known as "hyperstoichiometry" depends on the size of the nanoparticles. With particles that have a diameter around 10 nm, the ratio can reach between 1.1:1 and 1.7:1, depending on the mercury counterion. In these cases, the reaction is clearly occurring differently than it does with silver particles of "normal" size. The researchers postulate that the initially produced silver ions are absorbed into the silver nanoparticles and, under the catalytic influence of the tiny silver nanoparticles, are "recycled" back to elemental silver by the negatively charged counterions of the mercury salts, which in these experiments were nitrate or acetate. It has often been observed that very small nanoparticles have a higher catalytic activity than larger ones because their surface properties dominate over their bulk properties. The hyperstoichiometric effect suggests new approaches for the purification of runoff as well as catalysis.
To produce the necessary extremely small silver nanoparticles, the scientists equipped a silicon dioxide surface with individual silicon hydride (-SiH) groups. These are able to reduce silver ions to neutral silver atoms, which are bound to the surface and probably act as nucleation sites for the further aggregation of silver. The density of SiH groups and reaction time can be used to control the size of the particles. In contrast to conventional processes, this requires no stabilizers, which stick to the silver nanoparticles and alter their physical and chemical properties.
Dr. Kseniia Katok is a Marie Curie International Incoming Fellow at the University of Brighton (UK) hosted with the Nanoscience and Nanotechnology Group. She is particularly interested in the chemistry of silica and carbon and the development of innovative materials for applications in the environmental and biomedical sectors.
Further Information:
Dr. Kseniia V. Katok, Dr. Raymond L. D. Whitby, Dr. Takahiro Fukuda, Prof. Toru Maekawa, Dr. Igor Bezverkhyy, Prof. Sergey V. Mikhalovsky, Prof. Andrew B. Cundy:
Hyperstoichiometric Interaction Between Silver and Mercury at the Nanoscale.
In: Angewandte Chemie International Edition; article first published online: 3 February 2012, DOI 10.1002/anie.201106776
Source: Angewandte Chemie International Edition, press release 06/2012
Last update: 17.02.2012
Perma link: https://www.internetchemistry.com/news/2012/feb12/hyperstoichiometry.php
More chemistry: index | chemicals | lab equipment | job vacancies | sitemap
Internetchemistry: home | about | contact | imprint | privacy
© 1996 - 2023 Internetchemistry
