It is safe to say that no one in the whole world knows as much about the element boron as the team of Professor Holger Braunschweig at the University of Würzburg: The progress of the Würzburg researchers in the boron chemistry is currently published in as many as three top journals
The chemical element boron never fails to surprise chemists. It is so electron-deficient that it forms highly unusual compounds with other elements. For this reason, many textbooks devote a separate chapter to boron.
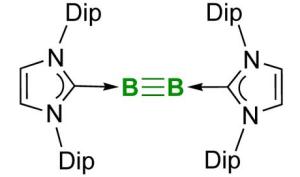
Stable boron-boron triple bond
The recent success in boron research published in the top journal "Science" by Professor Holger Braunschweig and his research group at the University of Würzburg is also worthy to be covered in textbooks: A team working with doctoral student Jan Mies succeeded for the first time in creating a stable chemical triple bond between two boron atoms.
Double or triple bonds can be formed by only a few other elements, such as carbon, silicon or nitrogen. These multiple bonds are of general interest, because they make interesting reactions possible, e.g. the synthesis of plastics like polyethylene. The triply linked boron compound might also open the way for the development of new materials and drugs.
In the past decades, many researchers failed to implement this elusive boron-boron triple bond. The Würzburg scientists, however, have achieved not only this. They also describe some examples of chemical reactions taking place at the triple bond. "This is the stuff that textbooks are made of. There is no doubt that the boron-boron triple bond will soon be introduced into the textbooks on inorganic chemistry": This is the opinion of an expert, who reviewed the Würzburg research on behalf of "Science".
Platinum at work
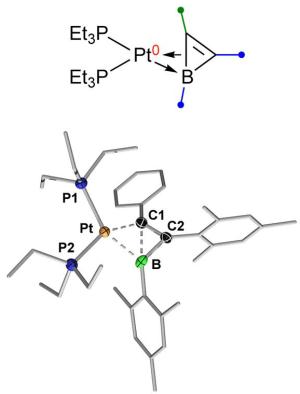
New ways of binding boron in a targeted way to itself or to other elements are described by the Würzburg chemists in two other current publications. In the journal "Nature Communications", they demonstrate the synthesis of a molecule, which they would never have expected to exist in stable form: In the relevant complex, a platinum atom has "half" broken the bond between boron and carbon.
Platinum is used as a catalyst in many industrial processes in order to increase the rate of chemical reactions. In this capacity, it can cause bonds between atoms to be formed or broken. "In our molecule, platinum has the effect that the bond between boron and carbon has a status that is somewhere between 'intact' and 'broken'," says doctoral student Bernd Pfaffinger, who was significantly involved in the synthesis of the molecule.
"We think that this represents a kind of snapshot of a process in which platinum is about to break a bond." Usually, such a state is far too volatile to be directly detected. So the stability of the molecule came as a complete surprise to the researchers.
Boron atoms linked into a chain
The journal "Nature Chemistry" finally presents a Würzburg study in which four boron atoms were linked into a chain. "Previously, such chain-linking could only be achieved with 'aggressive methods', involving high temperatures and explosive alkali metals like sodium," explains Braunschweig's doctoral student Qing Ye. His team has now succeeded for the first time in synthesizing the boron chains at room temperature in a carbon monoxide environment, i.e. under comparatively mild chemical conditions.
Thus, the Würzburg researchers have brought the synthesis of longer chains of boron atoms one step closer to realization. Scientists have high hopes for such boron polymers: These compounds are expected to have interesting electronic properties so they should be excellent materials for new applications in electronics.
Further Information:
Holger Braunschweig, Qing Ye, Alfredo Vargas, Rian D. Dewhurst, Krzysztof Radacki, Alexander Damme:
Controlled homocatenation of boron on a transition metal.
In: Nature Chemistry; published online17 June 2012, DOI 10.1038/nchem.1379
Holger Braunschweig, Rian D. Dewhurst, Kai Hammond, Jan Mies, Krzysztof Radacki, Alfredo Vargas:
Ambient-Temperature Isolation of a Compound with a Boron-Boron Triple Bond.
In: Science; Vol. 336 no. 6087 pp. 1420-1422, 15 June 2012, DOI 10.1126/science.1221138
Holger Braunschweig, Peter Brenner, Rian D. Dewhurst, Ivo Krummenacher, Bernd Pfaffinger, Alfredo Vargas:
Unsupported boron-carbon s-coordination to platinum as an isolable snapshot of s-bond activation.
In: Nature Communications; published 29 May 2012, DOI 10.1038/ncomms1884
Source: Julius-Maximilians University, Würzburg, Germany
Last update: 19.06.2012
Perma link: https://www.internetchemistry.com/news/2012/jun12/boron-boron-triple-bond.php
More chemistry: index | chemicals | lab equipment | job vacancies | sitemap
Internetchemistry: home | about | contact | imprint | privacy
© 1996 - 2023 Internetchemistry
