It is not always easy to distinguish between images and mirror images of molecules, but this knowledge is important when one image of a molecule is a drug and the mirror image is toxic.
One new approach to this may be chiral recognition in the gas phase. This involves using synchrotron radiation (highly energetic photons from a particle accelerator) to eject electrons from the molecules and analyzing their trajectories.
In the journal Angewandte Chemie, German researchers have now demonstrated that such experiments also work with a compact laser system [see below].
The trick is to replace the individual high-energy photon with three laser photons that excite the molecule through intermediate levels until it releases an electron (this method is known as REMPI, Resonance-Enhanced Multi-Photon Ionization). "It is thus possible to eject electrons with less energetic but more intense light," explains Thomas Baumert of the University of Kassel.
For the measurements, the light must be circularly polarized. What does this mean? "Ordinary" light consists of waves that oscillate in all spatial directions perpendicular to their direction of travel. If light is linearly polarized, the light waves oscillate exclusively in one plane. When light is circularly polarized, the light wave oscillates in a helical form, because its amplitude describes a circle around the axis of travel - either to the right or the left.
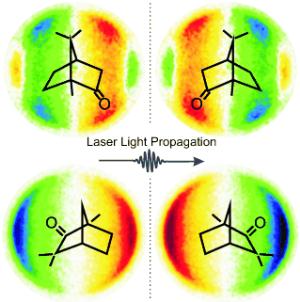
Molecules in the gas phase are randomly oriented and thus encounter the laser light from all possible angles; the ejected electrons also fly off in every possible direction as they leave the molecule. By using both a special configuration for measurement and special calculation processes, the team is able to determine the distribution of the angles of the electrons" flight paths. In the case of linearly polarized light, the distribution is symmetrical. "However, when the electrons are ejected by circularly polarized light, we find a distinct asymmetry to the angles at which the free electrons are found in relation to the laser beam," reports Baumert. "This asymmetry is inverted if left circularly polarized light is used instead of right, an effect known as photoelectron circular dichroism. We observe the same effect when we keep the circular polarization the same but change from the "right handed" to the "left handed" structure of the chiral molecule being observed." The researchers were able to demonstrate this with the chiral compounds camphor and fenchone.
"This circular dichroism effect has previously only been observed with synchrotron radiation. In contrast, our procedure uses a compact laser system, so that this method is not limited to basic laboratory research but, because of the magnitude of the observed effects, may also find its way into analysis," according to Baumert.
Thomas Baumert (Dr.) has been Professor of Experimental Physics at the University of Kassel for over a decade. His research interests include femtosecond spectroscopy and ultrasound control of matter by means of tailored light fields.
Further Information:
Christian Lux, Prof. Dr. Matthias Wollenhaupt, Tom Bolze, Dr. Qingqing Liang, Jens Köhler, Cristian Sarpe, Prof. Dr. Thomas Baumert:
Circular Dichroism in the Photoelectron Angular Distributions of Camphor and Fenchone from Multiphoton Ionization with Femtosecond Laser Pulses.
In: Angewandte Chemie International Edition; published online: 20 February 2012, DOI 10.1002/anie.201109035
Source: Angewandte Chemie International Edition, press release 09/2012
Last update: 06.03.2012
Perma link: https://www.internetchemistry.com/news/2012/mar12/chiral-molecule-detection.php
More chemistry: index | chemicals | lab equipment | job vacancies | sitemap
Internetchemistry: home | about | contact | imprint | privacy
© 1996 - 2023 Internetchemistry
