MEDFORD/SOMERVILLE, Mass. - A team of researchers at Tufts University's School of Arts and Sciences and School of Engineering have discovered that individual atoms can catalyze industrially important chemical reactions such as the hydrogenation of acetylene, offering potentially significant economic and environmental benefits.
The research appeared in the March 9, 2012 issue of Science [see below].
Hydrogenation - the addition of hydrogen atoms to an organic compound - is critical to the food, petrochemical and pharmaceutical industries. Hydrogenation requires the presence of a catalyst, usually a metal or an alloy of both precious and common metals, that allows the hydrogen atoms to bind with other molecules.
It is difficult to produce alloys that are selective hydrogenation catalysts, able to attach the hydrogen atoms to specific sites of another molecule.
Tufts chemists and chemical engineers reported that when single atoms of palladium, an expensive precious metal, were added to copper, which is much cheaper and readily available, the resulting "single atom alloy" became active and selective for hydrogenation reactions.
This is the first published research to directly relate the arrangement of individual atoms in a metal alloy to their ability to catalyze hydrogenation reactions, according to E. Charles H. Sykes, associate professor of chemistry at Tufts and senior author on the paper. Sykes focuses much of his research on single molecule chemistry.
Industrial processes typically use small clumps of precious metal five to 10 nanometers wide on supports to make a catalyst. The Tufts scientists scattered single atoms of palladium less than half a nanometer wide onto a copper support. With palladium costing about $650 per ounce, the single atom alloy approach offers big cost savings.
"The chemical reactions we're looking at with smaller amounts of palladium use less energy and yield less chemical byproduct waste, hence they're better for the environment," said Sykes. "These reactions are also more cost-effective because we're working with single atoms of precious metals, which is therefore much cheaper than big clusters of the material. Given that hydrogenation reactions are carried out on a scale of millions of tons per year, there is great potential for this new and less expensive type of catalytic surface."
For this research the Tufts team heated very small amounts of palladium to almost 1,000 C. At that temperature individual atoms evaporated and embedded themselves on the copper surface about three inches away.
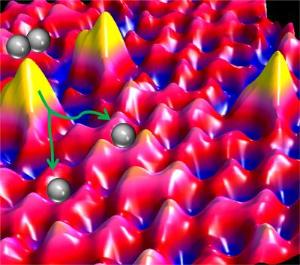
A scanning tunneling microscope, which records images of objects at the atomic level, enabled the team to see how these single atoms dispersed in the copper and how molecular hydrogen could then dissociate at individual, isolated palladium sites and spill over onto the copper surface layer.
"This is the first time there has been a definitive microscopic picture of the arrangement of atoms that promote a catalytic hydrogenation reaction. This picture is important because the catalytic hydrogenations that we're studying are vital to many industrial processes," added Sykes. "For example, in petroleum refining, catalytic hydrogenations are performed to make light and hydrogen-rich products like gasoline."
Georgios Kyriakou, research assistant professor of chemistry in the School of Arts and Sciences and first author of the paper; Maria Flytzani-Stephanopoulos, the Robert and Marcy Haber Endowed Professor in Energy Sustainability in the School of Engineering; and a joint Ph.D. student, Matthew Boucher, led testing that determined that the single atom alloy was more effective in catalyzing hydrogenation than denser mixtures of palladium and copper. Mass spectrometry showed that the new alloy catalyzed the hydrogenation of both styrene and acetylene with greater than 95% selectivity.
"With the rising cost of precious metals and the increasing scarcity of these metals, learning more about these reactions is encouraging in the search for sustainable global solutions," said Flytzani-Stephanopoulos. "We are looking at how these single-atom alloy catalysts could eventually be used as low-cost alternatives in hydrogenation and dehydrogenation processes for the production of 'green' agricultural chemicals, foods and pharmaceuticals," said Flytzani-Stephanopoulos.
This work was supported by the National Science Foundation and the U.S. Department of Energy as well as by Tufts Collaborates! funding.
Tufts University, located on three Massachusetts campuses in Boston, Medford/Somerville, and Grafton, and in Talloires, France, is recognized among the premier research universities in the United States. Tufts enjoys a global reputation for academic excellence and for the preparation of students as leaders in a wide range of professions. A growing number of innovative teaching and research initiatives span all campuses, and collaboration among the faculty and students in the undergraduate, graduate and professional programs across the university is widely encouraged.
Further Information:
Georgios Kyriakou, Matthew B. Boucher, April D. Jewell, Emily A. Lewis, Timothy J. Lawton, Ashleigh E. Baber, Heather L. Tierney, Maria Flytzani-Stephanopoulos, E. Charles H. Sykes:
Isolated Metal Atom Geometries as a Strategy for Selective Heterogeneous Hydrogenations.
In: Science; Vol. 335 no. 6073 pp. 1209 - 1212, 09 March 2012, DOI 10.1126/science.1215864
Source: Tufts University, USA
Last update: 08.03.2012
Perma link: https://www.internetchemistry.com/news/2012/mar12/single-atom-catalysts.php
More chemistry: index | chemicals | lab equipment | job vacancies | sitemap
Internetchemistry: home | about | contact | imprint | privacy
© 1996 - 2023 Internetchemistry
