From methanol to formaldehyde - this reaction is the starting point for the synthesis of many everyday plastics.
Using catalysts made of gold particles, formaldehyde could be produced without the environmentally hazardous waste generated in conventional methods.
Just how the mysterious gold catalyst works has been found out by theoretical and experimental researchers at the Ruhr-Universität Bochum in a cooperation project.
In the international edition of the journal 'Angewandte Chemie' [see below] they report in detail on what happens on the gold surface during the chemical reaction.
Gold should not really be suitable as a catalyst
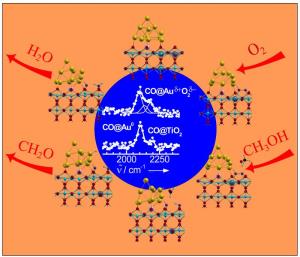
"That nanoparticles of gold actually selectively transform methanol into formaldehyde is remarkable", says Prof. Dr. Martin Muhler of the Laboratory of Industrial Chemistry at the RUB. "As a stable precious metal, gold should not really be suitable as a catalyst." However, gold particles of a few nanometres in size, anchored to a titanium dioxide surface, fulfil their purpose. You only need oxygen to set the reaction in motion, and the only waste product is water. How this is achieved is examined by Muhler"s team together with the groups of Prof. Dr. Dominik Marx of the Chair of Theoretical Chemistry and Dr. Yuemin Wang of the Department of Physical Chemistry I.
Oxygen binds at the interface between gold and titanium dioxide
The chemists identified the active site of the catalyst, i.e. the point at which the oxygen and methanol bind and are converted to water and formaldehyde. Elaborate calculations by Dr. Matteo Farnesi Camellone showed that oxygen binds at the interface between titanium dioxide and gold particles. Since titanium dioxide is a semiconductor, and thus electrically conductive, a charge exchange between oxygen, gold particles and titanium dioxide is possible here. Oxygen vacancies in the titanium dioxide further favour this charge transfer. Electrons transitionally transfer from the catalyst to the oxygen molecule. This allows the methanol to bind to the gold particles. In several further reaction steps, formaldehyde and water form. The solid, which consists of gold and titanium dioxide, is in the same state at the end of the reaction cycle as at the beginning, and is thus not consumed.
Experiment and theory: only the combination makes it possible
The RUB team clarified the individual reaction steps in detail. The researchers used computer simulations, so-called density functional calculations, and various spectroscopic techniques, namely, vibrational spectroscopy (HREELS method) and thermal desorption spectroscopy. In his model calculations, Dr. Farnesi quantified the charge exchange taking place during catalysis. Extremely sensitive vibrational spectroscopic measurements by Dr. Wang"s group confirmed the consequences of the charge transfer in the real system. "Through an intensive cooperation between theory and experiment, we have been able to qualitatively and quantitatively explore the active site and the entire reaction mechanism of this complex catalyst", stresses Prof. Marx.
Funding
The study originates from the Collaborative Research Centre 558 "Metal-substrate interactions in heterogeneous catalysis", which ended mid-2012. "The results are, so to speak, the crowning glory of the SFB works on alcohol oxidation", Muhler sums up. The project was further actively funded by the Cluster of Excellence "Ruhr Explores Solvation" RESOLV (EXC 1069), approved by the German Research Foundation (DFG) in 2012, in which researchers investigate the selective oxidation of alcohols in the liquid phase.
Further Information:
Dr. Matteo Farnesi Camellone, Dr. Jianli Zhao, Lanying Jin, Dr. Yuemin Wang, Prof. Dr. Martin Muhler, Prof. Dr. Dominik Marx:
Molecular Understanding of Reactivity and Selectivity for Methanol Oxidation at the Au/TiO2 Interface.
In: Angewandte Chemie International Edition; published online 15 April 2013, DOI 10.1002/anie.201301868
Source: Ruhr University, Bochum, Germany
Last update: 01.05.2013
Perma link: https://www.internetchemistry.com/news/2013/may13/au-tio2-catalyst.php
More chemistry: index | chemicals | lab equipment | job vacancies | sitemap
Internetchemistry: home | about | contact | imprint | privacy
© 1996 - 2023 Internetchemistry
