The Ancistrolikokines form a group of natural products, which is assigned to the naphthylisoquinoline alkaloids and were first isolated in 2000 from the Congolese Liana species Ancistrocladus likoko in the form of Ancistrolikokine representatives A to C [1]. At baseline, these compounds showed good to moderate activity against malaria when used in vitro against the NF54 and K1 strains of Plasmodium falciparum.
In the following years the discovery and characterization of other substances of this type followed, namely the ancistrolokokine D [2] and the ancistrolokokines C2, E, E2, F, G, H and H2 [3].
The newer ancistrolokokines show a moderate to good cytotoxic activity against the human pancreatic cancer cell line PANC-1 in a nutrient-poor medium - with simultaneously low toxicity. The Ancistrolikokine H2 showed the greatest effectiveness.
Ancistrolokokine E3
Recent research suggests that the ancistrolikokine variant E3 has promising activity against PANC-1 pancreatic carcinoma cells, known for their ability to thrive and form metastases even under extreme conditions with few nutrients and low oxygen levels [4]. This unusual resistance of cells to nutrient deficiency is one of the reasons why pancreatic cancer is so deadly: While most cells would die under these extreme conditions, pancreatic cancer cells survive by activating a cell signaling pathway called Akt / mTOR. Ancistrolikokine E3 is now able to inhibit this autophagy pathway of cancer cells.
For their studies, the researchers used the milled twigs of Liana Ancistrocladus likoko and separated the ingredients using high performance liquid chromatography HPLC. The alkaloid Ancistrolikokine E3 thus obtained was chemically characterized and its three-dimensional structure determined (see structural formula below).
The scientists found that this new drug killed the pancreatic cancer cells in a nutrient deficit - but not when the nutrients were abundant. Ancistrolikokine E3 also inhibited the migration and colonization of cancer cells in laboratory tests, suggesting that the drug may prevent metastasis in patients.
As a result, Ancistrolikokine E3 is a promising drug for the development of new cancer medicines.
Structural Formulas of Ancistrolikokine
The substances differ structurally in the substitution patterns of the hydroxy (-OH) and methoxy groups (-OCH3) and in the degree of hydrogenation of the quinoline ring. Common to all is the 5,8'-linkage of the isoquinoline ring with the substituted naphthalene function.

(1R,3R)-5-(4-Hydroxy-5-methoxy-7-methyl-1-naphthyl)-6-methoxy-1,2,3-trimethyl-1,2,3,4-tetrahydro-8-isoquinolinol
C25H29NO4
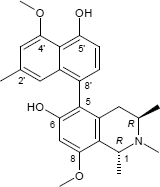
C25H29NO4
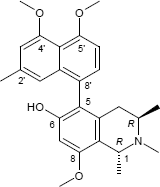
C26H31NO4
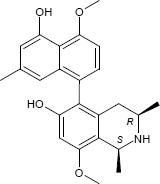
(1S,3R)-5-(5-Hydroxy-4-methoxy-7-methyl-1-naphthyl)-8-methoxy-1,3-dimethyl-1,2,3,4-tetrahydro-6-isoquinolinol
C24H27NO4
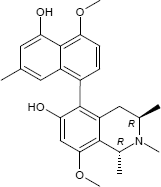
(1R,3R)-5-(5-Hydroxy-4-methoxy-7-methyl-1-naphthyl)-8-methoxy-1,2,3-trimethyl-1,2,3,4-tetrahydro-6-isoquinolinol
C25H29NO4
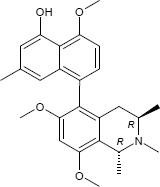
C26H31NO4
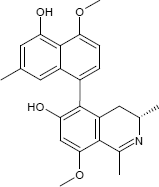
(3S)-5-(5-Hydroxy-4-methoxy-7-methyl-1-naphthyl)-8-methoxy-1,3-dimethyl-3,4-dihydro-6-isoquinolinol
C24H25NO4
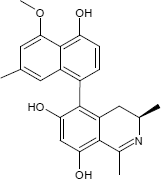
C23H23NO4
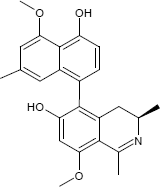
C24H25NO4
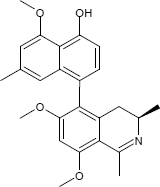
C25H27NO4
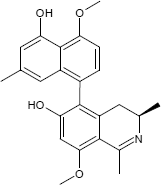
C24H25NO4
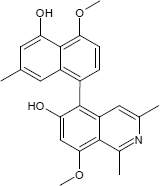
C24H23NO4
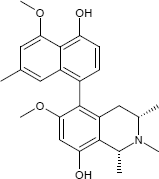
C25H29NO4
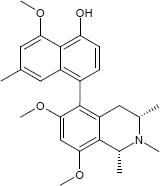
C26H31NO4
Literature sources and further information:
[1] - Gerhard Bringmann, Christian Günther, Wael Saeb, Jan Mies, Anura Wickramasinghe, Virima Mudogo, Reto Brun:
Ancistrolikokines A-C: New 5,8'-Coupled Naphthylisoquinoline Alkaloids from Ancistrocladus likoko.
In: Journal of Natural Products, (2000), DOI 10.1021/np000199t.
[2] - Gerhard Bringmann et al.:
Ancistrolikokine D, a 5,8'-coupled naphthylisoquinoline alkaloid, and related natural products from Ancistrocladus likoko.
In: Phytochemistry, (2003), DOI 10.1016/S0031-9422(02)00570-8.
[3] - Shaimaa Fayez, Doris Feineis, Virima Mudogo, Suresh Awale, Gerhard Bringmann:
Ancistrolikokines E-H and related 5,8'-coupled naphthylisoquinoline alkaloids from the Congolese liana Ancistrocladus likoko with antiausterity activities against PANC-1 human pancreatic cancer cells.
In: RCS Advances, (2017), DOI 10.1039/c7ra11200a.
[4] - Suresh Awale, Dya Fita Dibwe, Chandrasekar Balachandran, Shaimaa Fayez, Doris Feineis, Blaise Kimbadi Lombe, Gerhard Bringmann:
Ancistrolikokine E3, a 5,8'-Coupled Naphthylisoquinoline Alkaloid, Eliminates the Tolerance of Cancer Cells to Nutrition Starvation by Inhibition of the Akt/mTOR/Autophagy Signaling Pathway.
In: Journal of Natural Products, (2018), DOI 10.1021/acs.jnatprod.8b00733.
Category: compound classes
Last update: 11 November 2022
Perma link: https://www.internetchemistry.com/compound-classes/a/ancistrolikokines.php
More chemistry: index | chemicals | lab equipment | job vacancies | sitemap
Internetchemistry: home | about | contact | imprint | privacy
© 1996 - 2023 Internetchemistry
