The acenes form a series of cyclic, linearly condensed, aromatic hydrocarbons [see figure]; the molecular structure consists of planar sets of linearly fused benzene rings:
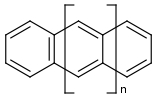
The first two members are naphthalene (n=0) and anthracene (n=1). The names of the higher homologous acenes are formed from the Greek numerals (tetra-, penta, hexa, etc. = number of benzene rings) and the ending -acene (elusion of the 2nd letter a): tetracene, pentacene, hexacene, ... [1]
The higher representatives are also referred to as polyacenes; due to the increasing size of the conjugated π-electron system, these are - like their derivatives - in the interest of research as potential organic semiconductors for electrotechnical and molecular electronic applications.
However, the synthesis of the higher acenes is more challenging because of their unstable nature: each acene has only one aromatic sextet distributed throughout the conjugated system (Clar sextet). This leads to a rapid narrowing of the HOMO–LUMO gap and an increase in chemical reactivity with each additional fused ring. Since the reactivity is significantly influenced by substituents, a number of functionalization strategies have been developed to circumvent the instability of acenes [2].
Literature sources and further information:
[1] - Fused Ring and Bridged Fused Ring Nomenclature (IUPAC Recommendations).
[2] - Ruth Dorel, Antonio M. Echavarren
Strategies for the Synthesis of Higher Acenes.
In: European Journal of Organic Chemistry, (2016], DOI 10.1002/ejoc.201601129, open access.
Category: compound classes
Last update: 06 October 2022
Perma link: https://www.internetchemistry.com/compound-classes/a/acenes.php
More chemistry: index | chemicals | lab equipment | job vacancies | sitemap
Internetchemistry: home | about | contact | imprint | privacy
© 1996 - 2023 Internetchemistry
